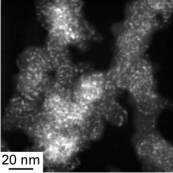
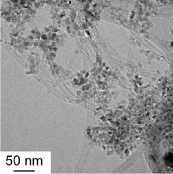
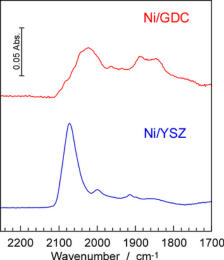
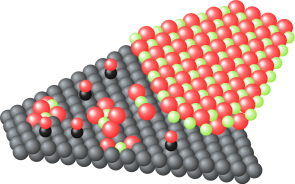
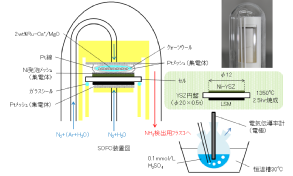
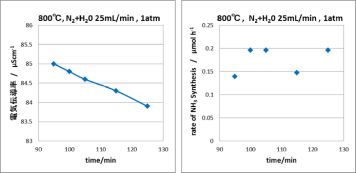
SOFCs has been commercialized in residential cogenerationsystems. New model of ENE-FARM, which is ahousecogenerationsystem generating electricity (45% efficiency) and supplying hot water (43% efficiency). Total efficiency reaches 88%, which obviouslyovercomes any large-scale thermal power plant with total energy efficiency from the fuel. One of the advantages of SOFCs is thatthe SOFCs can directly utilize various kinds of fuels such as LNG and syngas without a reforming reactor, but not onlyhydrogen.However, direct use of such carbon based fuels frequently causes carbon deposition on anode electrocatalysts of Ni, so that thecritical control of steam ratio in the fuel is required. Interestingly, the nature of carbondepositionon Ni has been known to bestrongly affected by the kinds of oxide electrolytes, even if the carbon deposition occurs onthe Nisurface.
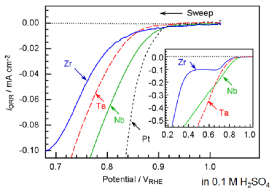
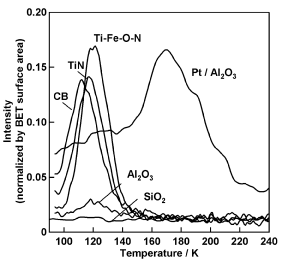
We study the mechanism of carbon deposition using infrared spectroscopy and reveal the function of electrolytesincarbondeposition. We also study the adsorption sites of oxygen molecules on cathode electrocatalysts of SOFCs such as La-Sr-Mnoxides. |